ctx clinical trial
This is an open-label multicenter Phase 1 study evaluating the safety and efficacy of CTX110 in subjects with relapsed or refractory B-cell malignancies. When tested in human myeloid cell lines K562 and MV-4-11 CTX-712 showed a strong inhibitory effect on cell proliferation IC 50 015 and 0036 μM respectively.
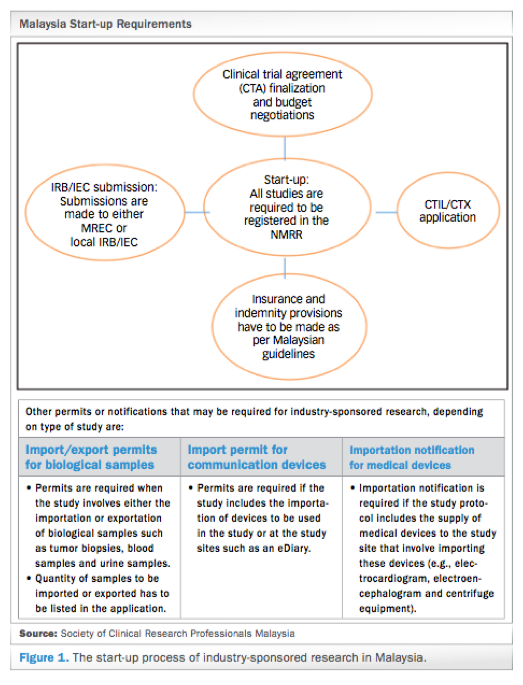
Malaysia S Clinical Research Ecosystem
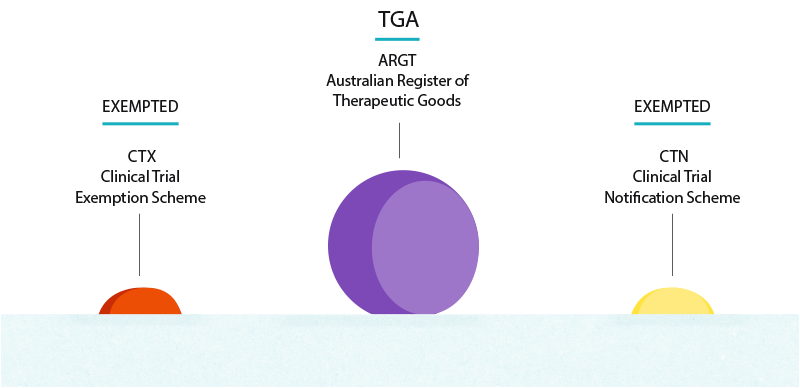
CTX Clinical Trial Exemption An approval process.

. In the RESTORE study doctors will look at markers of CTX in the urine to see if they are lowered when CDCA is used to treat CTX. Clinical trials of medicines and biologicals regulated under the CTN or CTA schemes are subject to the TGAs Good Clinical Practice GCP Inspection Program. Clinical Trials Xpress CTX is an initiative of the University of Texas System established to provide an efficient and scalable centralized operating model.
The ongoing Phase 12 open-label trial CLIMB-SCD-121 is designed to assess the safety and efficacy of a single dose of CTX001 in patients ages 12 to 35 with severe SCD. The Therapeutic Goods Administration TGA directly reviews the planned clinical trial and must give their approval for the clinical trial to go ahead. Study Design Go to Resource links provided by the National Library of Medicine.
None Open Label Primary Purpose. The study will evaluate the safety and efficacy of autologous CRISPR-Cas9 Modified CD34 Human Hematopoietic Stem and Progenitor Cells hHSPCs using CTX001. Eligible participants pediatric and adult will need to visit a study center about 18 times.
The study will last approximately 25 to 28 weeks 6-7 months. In people with CTX the body is unable to break down cholesterol properly causing toxins eg cholestanol and bile alcohols to build up throughout the body over time. Interventional Clinical Trial Estimated Enrollment.
This is a single-arm open-label multicenter Phase 1 study evaluating the safety and efficacy of CTX130 in subjects with relapsed or refractory renal cell carcinoma. Join our mailing list to receive information and news as we begin to gather and expand the CTX community. The trial will enroll up to 45 patients and follow patients for approximately two years after infusion.
Doctors will also look at the safety and potential side effects of CDCA treatment. The study has 2 groups. The CTN pathway is by far the most frequent regulatory pathway in.
Current CTX Clinical Trials. Clinical diagnosis of CTX with biochemical confirmation. The TGA has published guidance on the Good Clinical Practice GCP Inspection program to provide sponsors with further information about the programs scope and process.
The anti-leukemic effect was also confirmed by survival assay using a total of 79 primary AML cells the average of IC 50 was 0078 μM. The CTX Alliance is a newly-formed patient organization solely dedicated to providing resources support and promoting research for CTX patients families and healthcare providers. There will also be times when participants or their caregivers will need to speak by telephone to the study team at a study center.
CTX is a rare progressive disorder that can affect the brain spinal cord tendons eyes and arteries. Join the CTX Alliance. A Phase 1 clinical trial of CTX-712 in solid tumors and hematological malignancies demonstrated a clinically acceptable safety profile.
Do You Have Cerebrotendinous Xanthomatosis CTX. As such CTX is a cryopreserved clinical and commercial-grade cell therapy product capable of treating all eligible patients presenting. Each patient will be asked to participate in a long-term follow-up trial.
All studies both privately and government funded are listed on clinicaltrialsgov. A Phase 123 Study of the Safety and Efficacy of a Single Dose of Autologous CRISPR-Cas9 Modified CD34 Human Hematopoietic Stem and. This is a single-arm open-label multicenter Phase 1 study evaluating the safety and efficacy of CTX120 in subjects with relapsed or refractory multiple myeloma.
Clinical Trial Exemption CTX scheme renamed as Clinical Trial Approval CTA scheme 6 November 2020 The Therapeutic Goods Administration TGA has changed the name of the Clinical Trial Exemption CTX scheme to the Clinical Trial Approval CTA scheme. Study Design Go to Resource links provided by the National Library of Medicine. Women of childbearing potential must agree to the use of one highly reliable method of contraception during the study plus one additional barrier method during sexual activity.
CEREBROTENDINOUS XANTHOMATOSIS CTX Cerebrotendinous xanthomatosis CTX is a rare progressive and underdiagnosed bile acid synthesis disorder affecting many parts of the body. Since the last publication of Guideline for the application of Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX 5th Edition in 2009 we have witnessed robust growth in clinical research industry with the aim to achieve at least 1000 clinical trials to generate GNI of RM5784 million by the year 2020 in Malaysia. CTX has been shown to be safe and well-tolerated in a first-in-man UK clinical trial PISCES I in eleven disabled stroke patients who were followed up for at least two years post-treatment.
The study may enroll up to 143 subjects in total. Clinical trials CX-2009-002 a Phase 2 multi-arm study is now enrolling patients with human epidermal growth factor receptor 2 HER2-non-amplified breast cancer. Clinical Trials Patient Registry - Cerebrotendinous Xanthomatosis CTX Clinical Trials Patient Registry Clinical Research The investigational therapies explored in clinical trials are key to improved therapies among the whole CTX community.
This is a single-arm open-label multi-site single-dose Phase 123 study in subjects with severe sickle cell disease SCD. Vertex-CRISPRs CTX001 shows positive outcomes in trial patients Vertex Pharmaceuticals and CRISPR Therapeutics have reported positive interim results from two Phase III clinical trials of investigational ex-vivo CRISPRCas9 gene-edited therapy CTX001. As for antitumor efficacy it was observed in multiple subjects establishing an initial Proof of Concept POC.
Gene-edited therapy shows promise in patients suffering from severe hemoglobinopathies. Chenodal is not indicated for the treatment of CTX but has received a medical necessity determination in the US by the FDA. Adult 16 years of age and older and Pediatric under 16 years of age.
The study may enroll approximately 80 subjects in total. The study may enroll approximately 107subjects in total. Travere Therapeutics is conducting a Phase 3 clinical trial to examine the safety and efficacy of Chenodal to treat CTX.
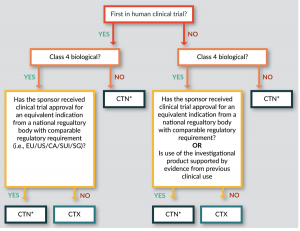
Bioinsights The Regulatory Environment For Cell Therapies In Australia An Opportunity To Expedite Clinical Development

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini
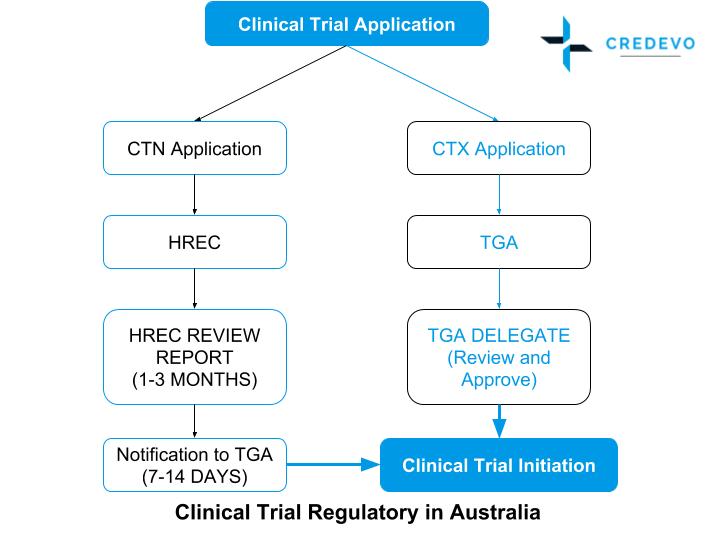
How To Get Started With Your Clinical Trials In Australia

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini

Regulatory Requirements For Clinical Trials Australia Vs The Us

Clinical Trials Medical Device Trials Genesis Research Services
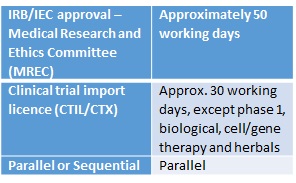
Regulatory Timelines In The Asia Pacific George Clinical
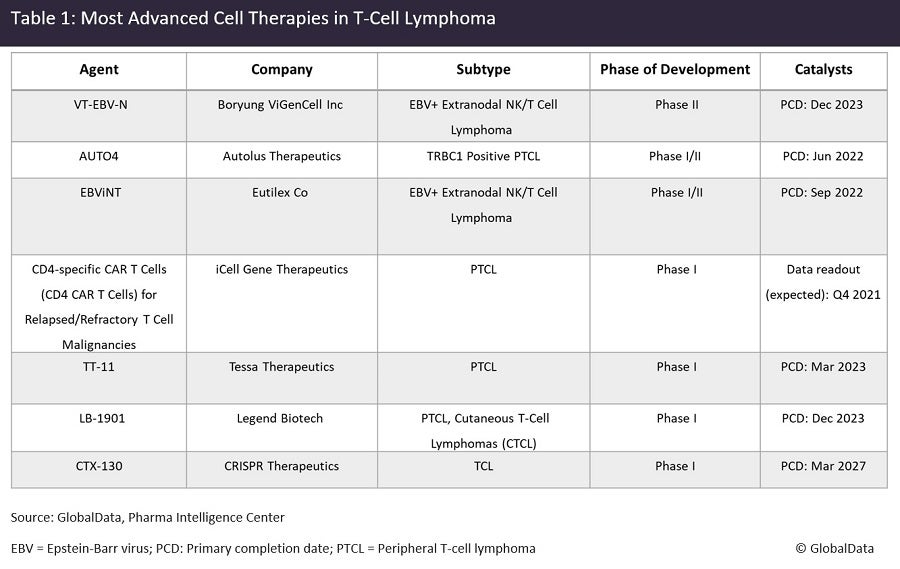
The Clinical Trial Landscape Of Cell Therapies For T Cell Lymphomas

Clinical Trials Medical Device Trials Genesis Research Services

Clinical Trials Xpress Patient Recruitment Technology Symposium Institute For Integration Of Medicine And Science

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini

Regulatory Timelines In The Asia Pacific

Restore Study Cerebrotendinous Xanthomatosis Ctx Research Study
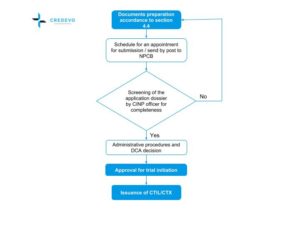
Clinical Trials In Malaysia Why And How To Start Credevo Articles

Ut System Clinical Trials Xpress Ctx Helpful For Cprit Mira Grants Institute For Integration Of Medicine And Science

Comments
Post a Comment